30 years of LORENZ (part 5)
Posted on February 15, 2021
Finger on the pulse: LORENZ brings innovation to the Regulatory Affairs world
The continuous technical advances in the digital world also bring new challenges. Efficient data and process management and the integration of process steps are becoming ever more complex. Over the past three decades, LORENZ has continuously taken the latest technical advances and trends and applied them to the specialized world of regulatory affairs.

Automation
One of these key trends is the automation of standardized processes, to save time and money and to avoid mistakes. There is plenty of potential for errors in the regulatory affairs world: a submission can now exceed a million pages, driven by ever more complex regulations and specifications. In parallel, many health authorities now have their own communication portals (gateways), so the sheer volume of communication back and forth with the agency - and often with third parties involved in the submission - means there are many more 'to-dos' landing on many more desks.
To ease these processes, we launched LORENZ Automator, which can take over many rule-based work sequences that arise in the regulatory affairs context. From the synchronization of master data between systems to the automation of complex workflows, the routine tasks are taken care of reliably, so human brainpower can concentrate on more important work.
Have you met Adam, your virtual regulatory assistant?
The LORENZ team is always eager to find new ways to make work in the RA world easier through the use of new technologies. So, where you have Siri, Alexa or Google in the more general area, for regulatory affairs we offer you ADAM.
This virtual chat-based assistant is an integral part of LORENZ Automator. You can ask your 'virtual colleague' to do a specific task such as to start sending a submission sequence to the EMA, or to show a certain report in LORENZ drugTrack.
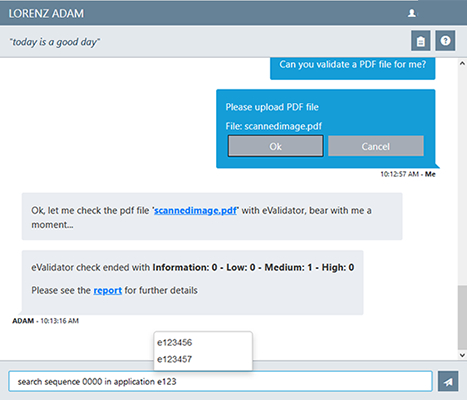
ADAM, the virtual chat-based assistant (LORENZ Automator)
Interoperability and RIM - a small word, a big challenge
To further improve efficiency in regulatory processes, LORENZ realized early on that interoperability - meaning the exchange of data across different systems - is a vitally important concept.
Since the xEVMPD standard was implemented in 2012, LORENZ drugTrack, our product information and lifecycle management software, has been working for marketing authorization holders as one of the central databases in the regulatory process. It offers a variety of important reports which also include data from other software.
In general, every LORENZ product is engineered to operate seamlessly with every other product, as well as with many third-party solutions. It’s all part of our approach to providing the platform for configuring your ideal Regulatory Information Management solution, even if you happen to use another provider for a specific function. That results in true flexibility for our customers! Over the past years we have therefore moved our business capabilities, such as Submission Management (LORENZ docuBridge) and Product Information and Product Lifecycle Management (LORENZ drugTrack), onto a core central system called "LORENZ Foundation". Through the LORENZ Foundation, both LORENZ products and third-party tools can be interconnected so that they interact with each other effectively. With this approach, we offer our customers the greatest possible flexibility and individuality for their RIM solution.
This is important because E2E (end-to-end) regulatory information management (RIM) will always be a moving target. Many organizational and technological factors will affect your skill requirements. Increasingly, RIM is seen as an integrated end-to-end process. Each element along the chain has a vital role to play, from the compilation and publishing processes on to document management and conforming with relevant technical regulatory requirements. In the end, maximizing access to information is what will enable industry to gain efficiencies and remain competitive. This is the point where LORENZ steps in.
The future: Let's simplify your work
LORENZ is heavily focused on regulatory matters, since this is our expertise. But we also aim to bring the vast array of technical innovations that are available today into the complex world of regulatory affairs, thus simplifying your work.
Just like we have done over the last 30 years, we will continue to monitor the market closely, working to understand your needs and to provide you with the right support. Our ultimate goal remains the same: to increase the efficiency of your own daily operations. In the e-regulation software field, we all benefit when working relationships are solid and long-term. They enable us to learn from each other and, in the process, to increase the effectiveness of our products and services. LORENZ would like to take this opportunity to thank all of our customers from agencies and from industry for 30 years of outstanding collaboration, for the open exchange of information, and for the trust you have placed in LORENZ during our journey together.
Let's see what comes next ... LORENZ is here for you!

Your LORENZ Life Sciences Group Management Team
All articles about LORENZ's anniversary:
30 years of LORENZ (part 1)
30 years of LORENZ (part 2)
30 years of LORENZ (part 3)
30 years of LORENZ (part 4)
This entry was posted in LORENZ News.

