drugTrack is a versatile system for keeping track of all your product information and managing all regulatory activities in the pharmaceutical product life cycle. Find the information you need and generate management reports on-the-fly. With this regulatory lifecycle management tool, you can prepare for IDMP/SPOR compliance.
Find out what LORENZ drugTrack can do for you
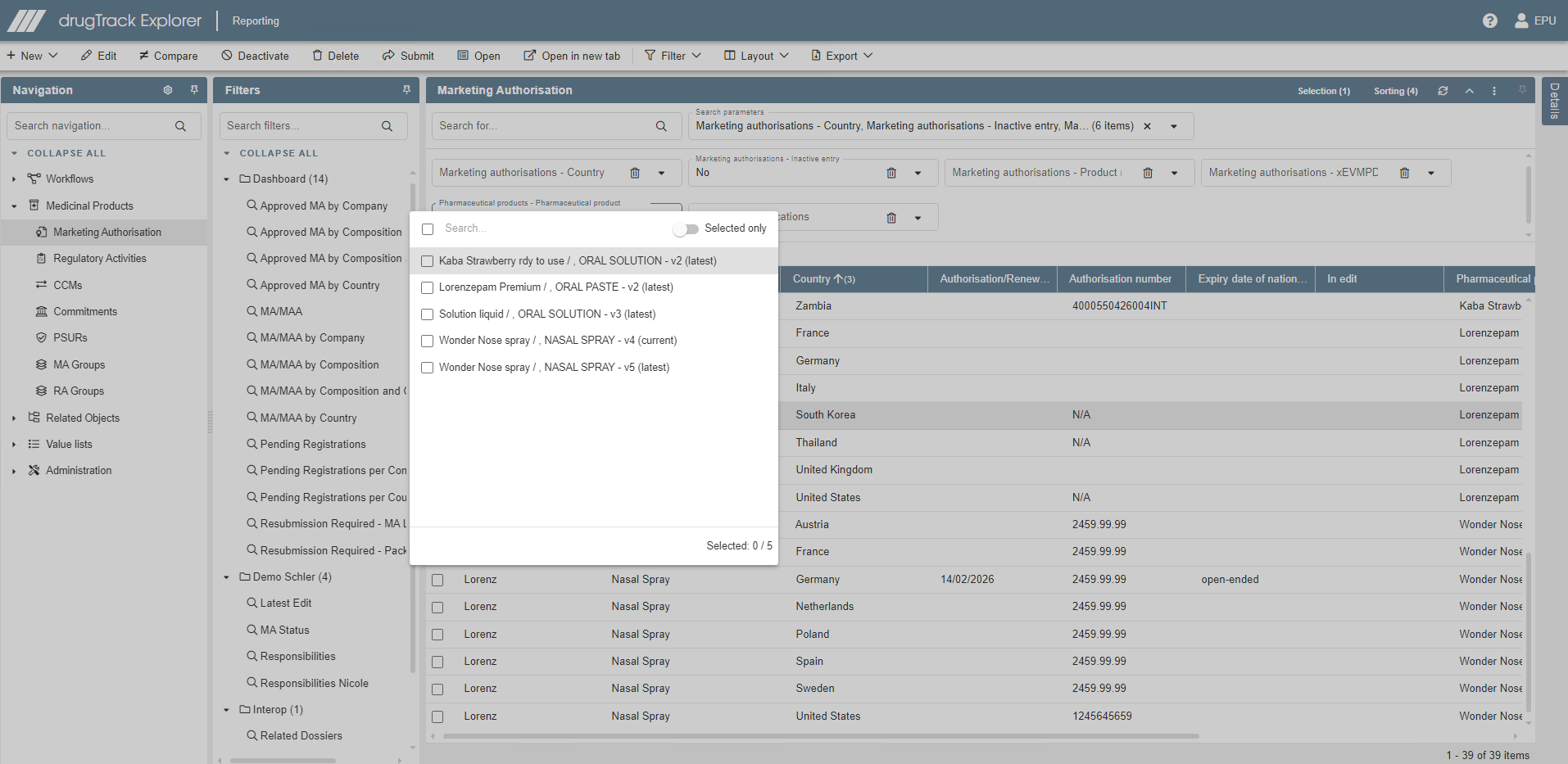
Product information database
Effortlessly create, update and maintain medicinal product, medical device, development product and drug master file information
Interoperability
Work flawlessly with other LORENZ solutions and third-party software
Product lifecycle management
Track the entire pharmaceutical product life cycle using regulatory activities and change control management
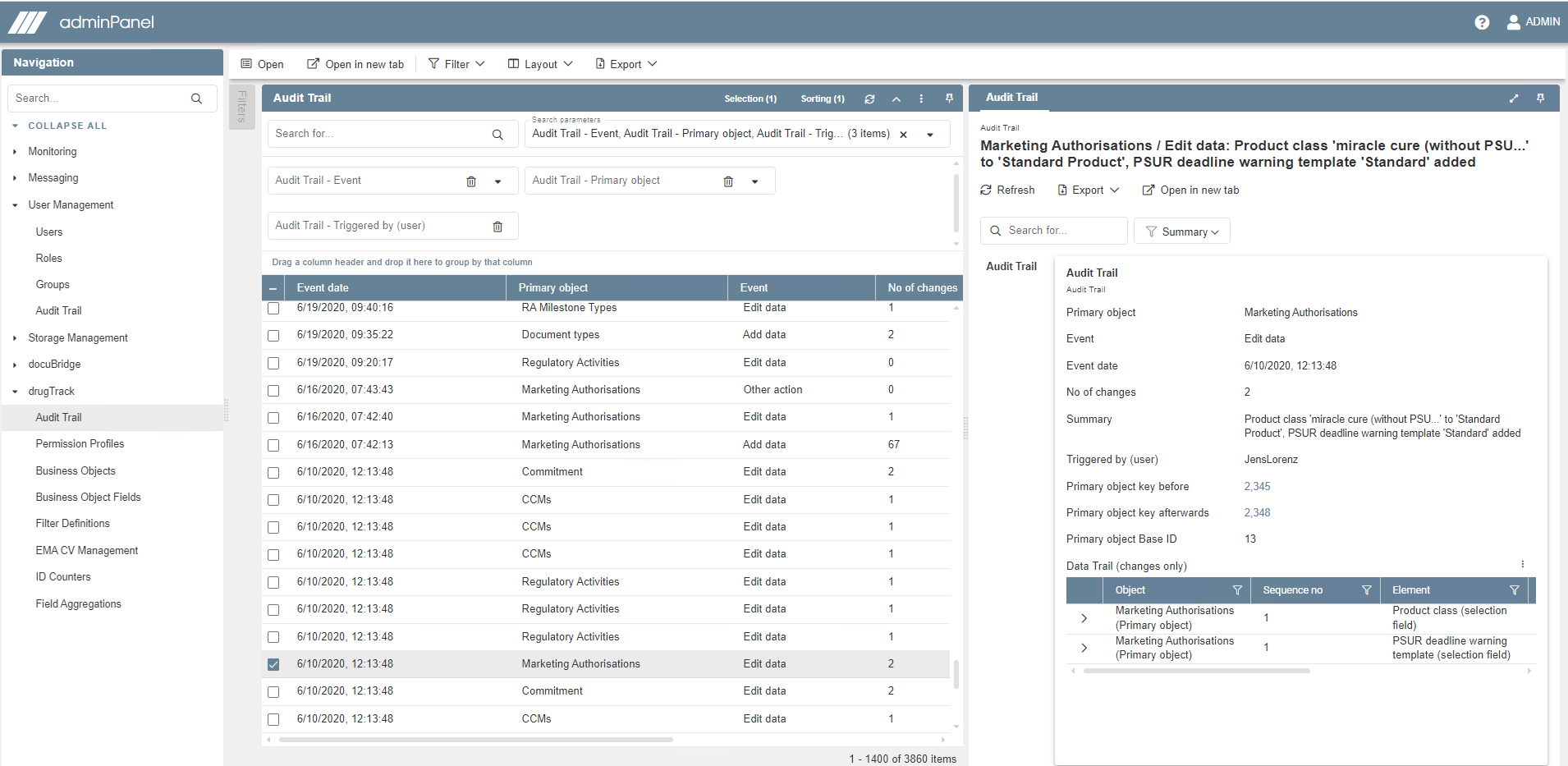
Compliance management
Ensure quality control with four-eyes principle, audit trail, deadline triggers, reminders and e-mail notifications
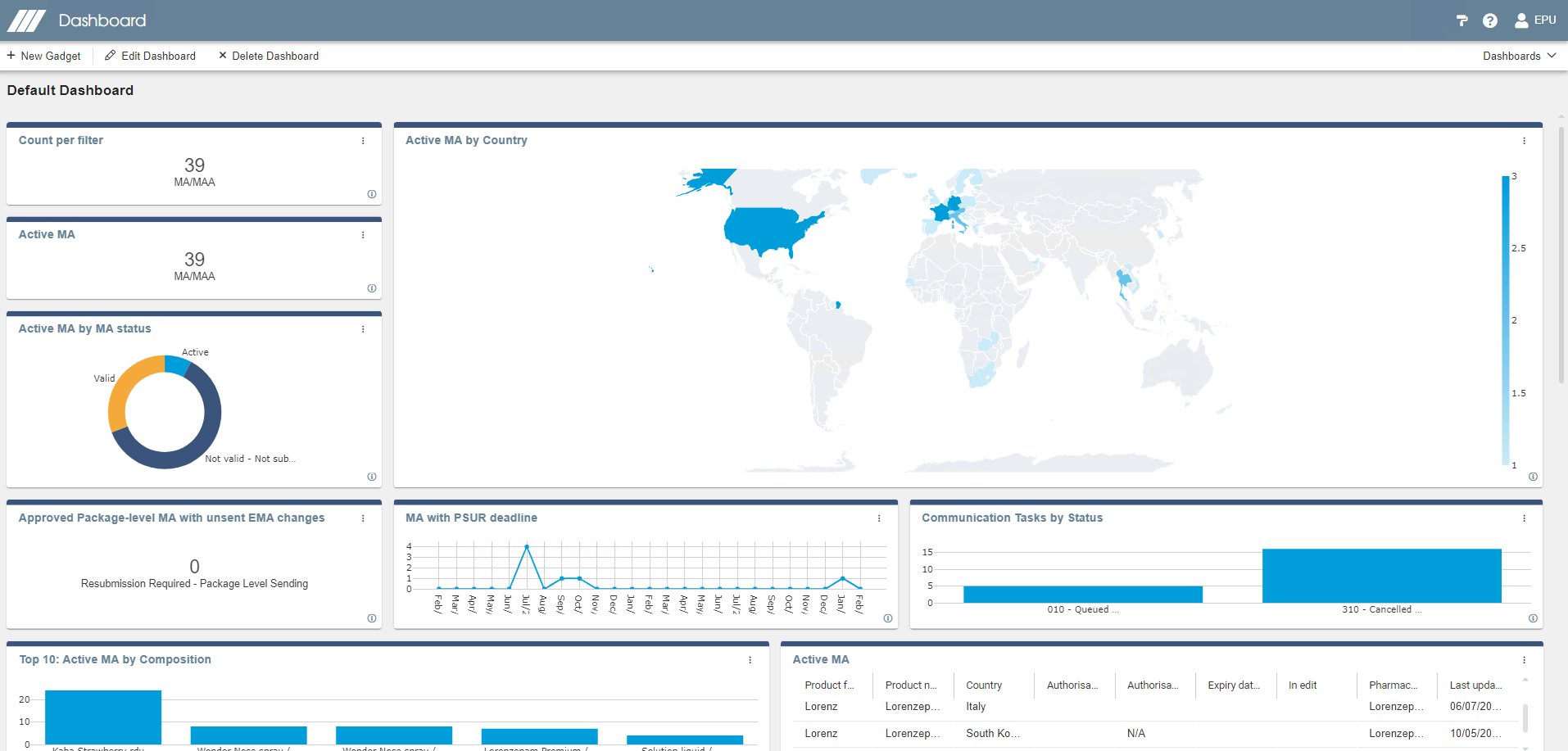
Querying, reports & dashboard
Illustrate your data with dashboard graphics. Create your own dashboard and export information using different formats
xEVMPD/IDMP compliance
Create and update EudraVigilance medicinal product report messages to the EMA. Prepare your data in compliance with the IDMP data model
Get a first glimpse of LORENZ drugTrack – an all-encompassing product information and life cycle management solution
LORENZ drugTrack as part of your RIMS
Connect drugTrack to other LORENZ and third-party software for a flexible Regulatory Information Management solution.
Rest easy knowing our team is dedicated to supporting your work with LORENZ drugTrack
Flexible training options
Get off to a good start and get the most out of the software. Individual trainings can be held online or at one of our facilities - or one of our trainers can come to your location. In addition, we also offer a range of webinars to refresh your knowledge.
Reliable release schedules
Rest assured that you are always working with the latest features of the software to make your job easier. We provide you with two major releases each year, in April and October, as well as minor and specific releases if necessary.
All-encompassing services
Rely on our team of experts for installation, validation, migration, configuration and customer support as well as consulting services to meet all your needs.