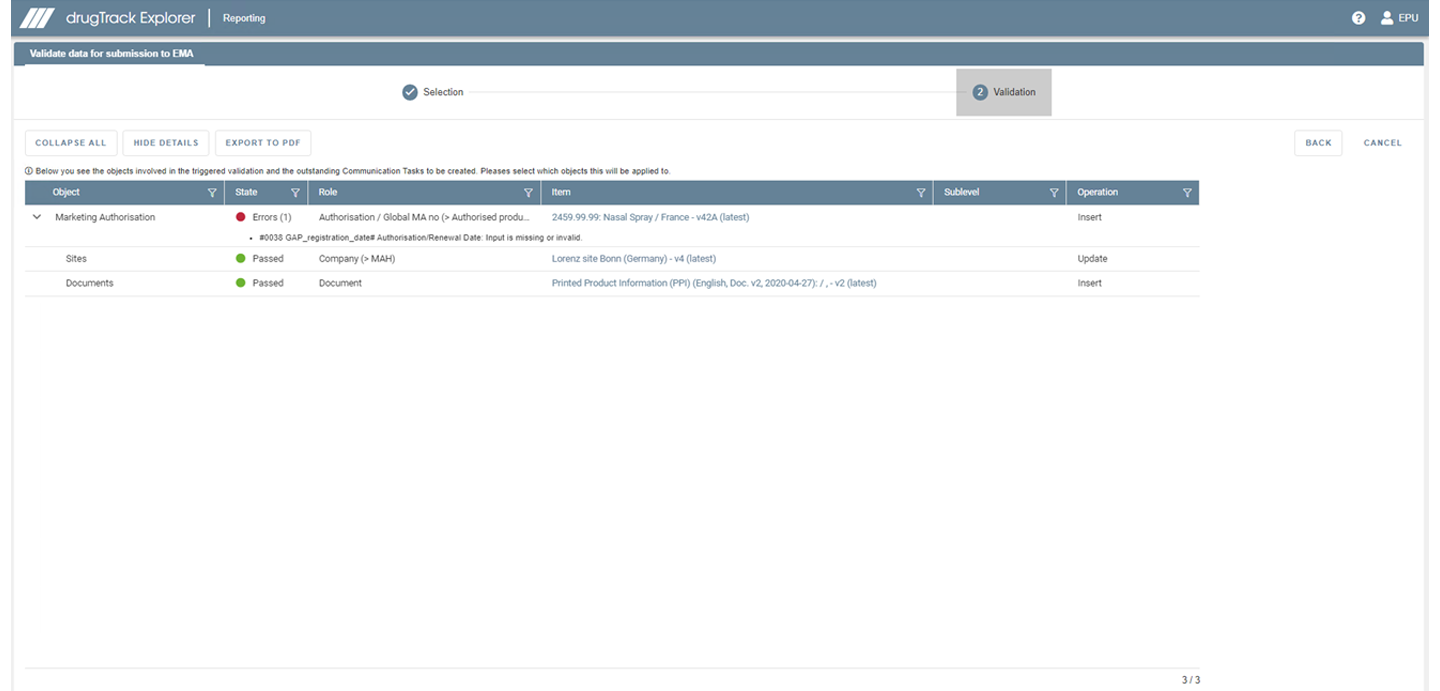
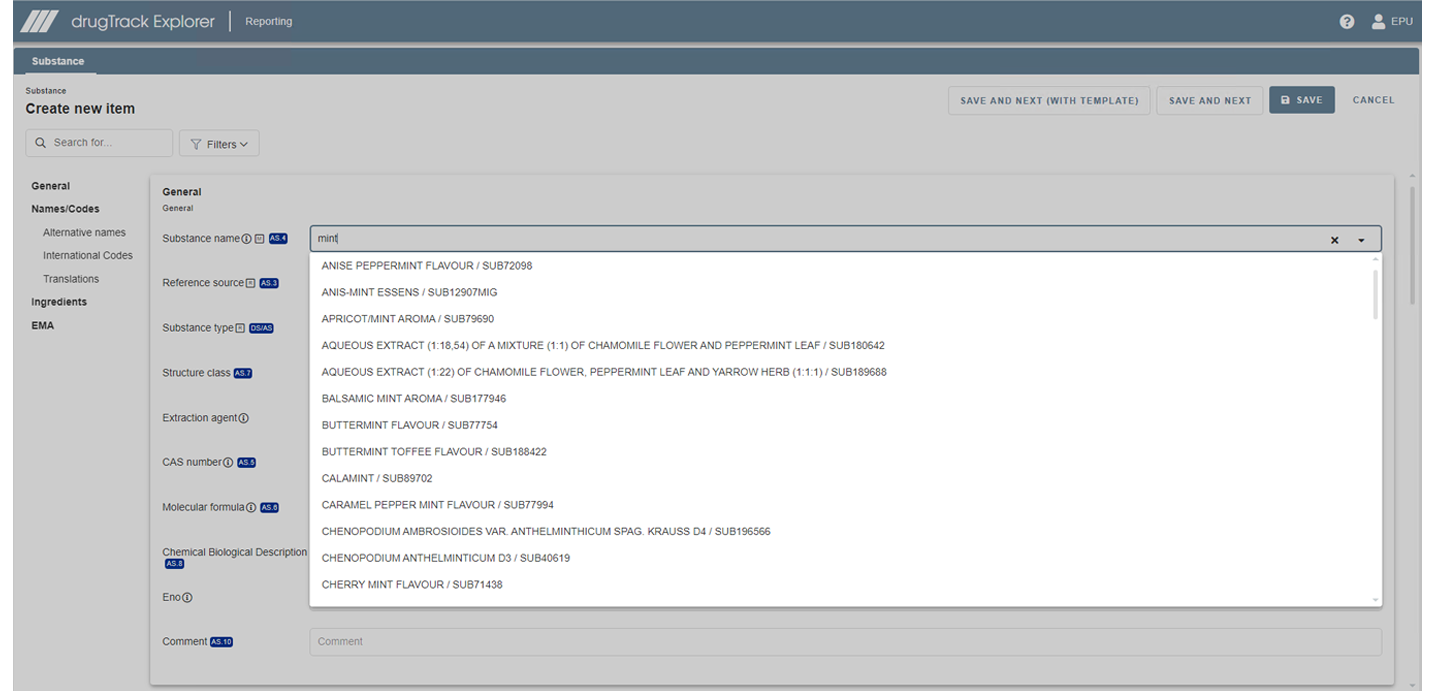
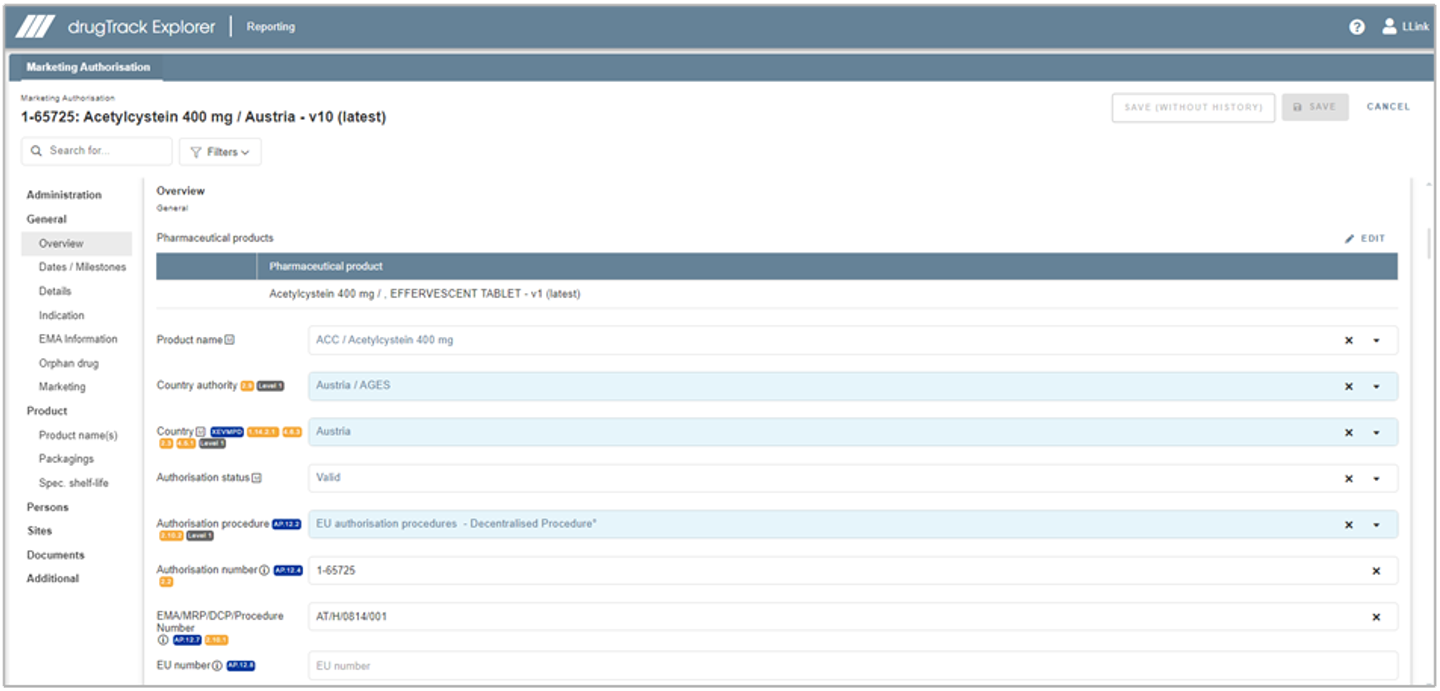
LORENZ drugTrack can help you prepare for IDMP by identifying and understanding compliance gaps and allowing you to already implement IDMP data in the system.
How drugTrack can help you with the transition to IDMP compliance
Database is ready for the first iteration of IDMP
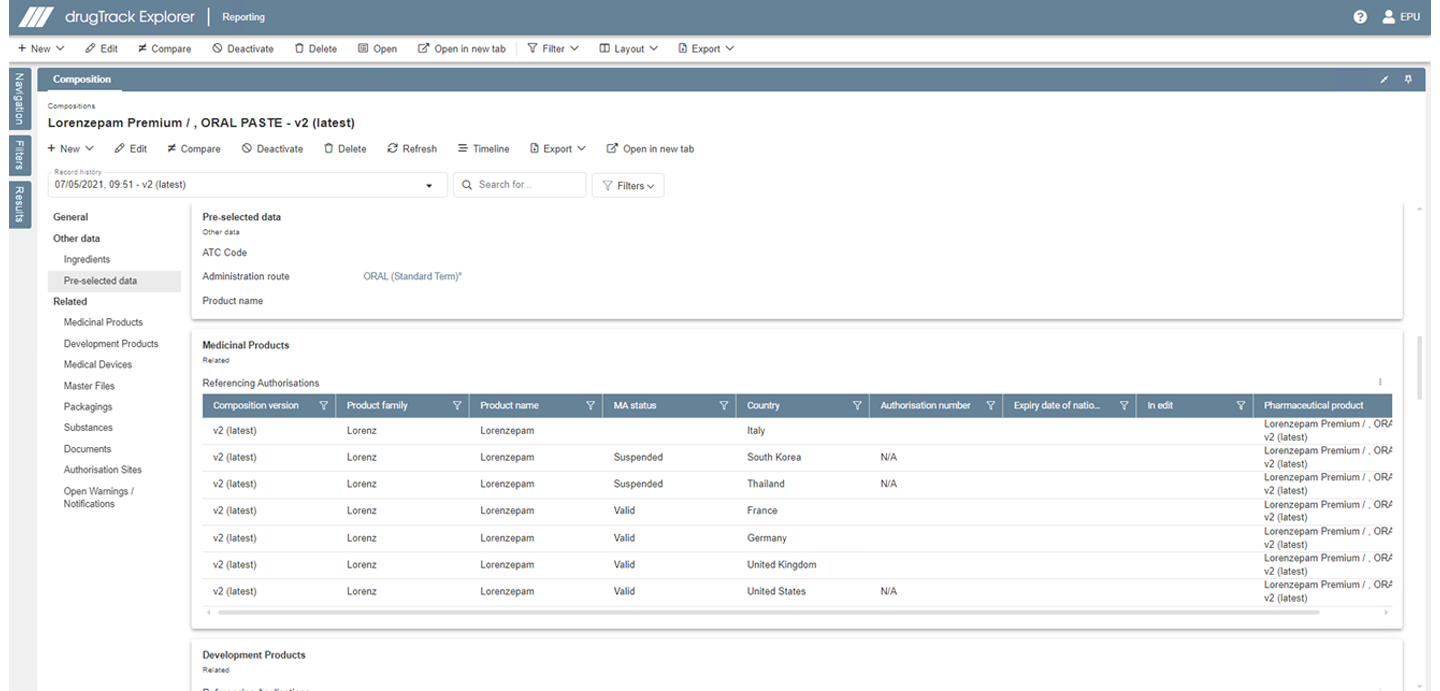
drugTrack's database already has all fields in place which will be necessary for the first iteration of IDMP.
Identify your missing data
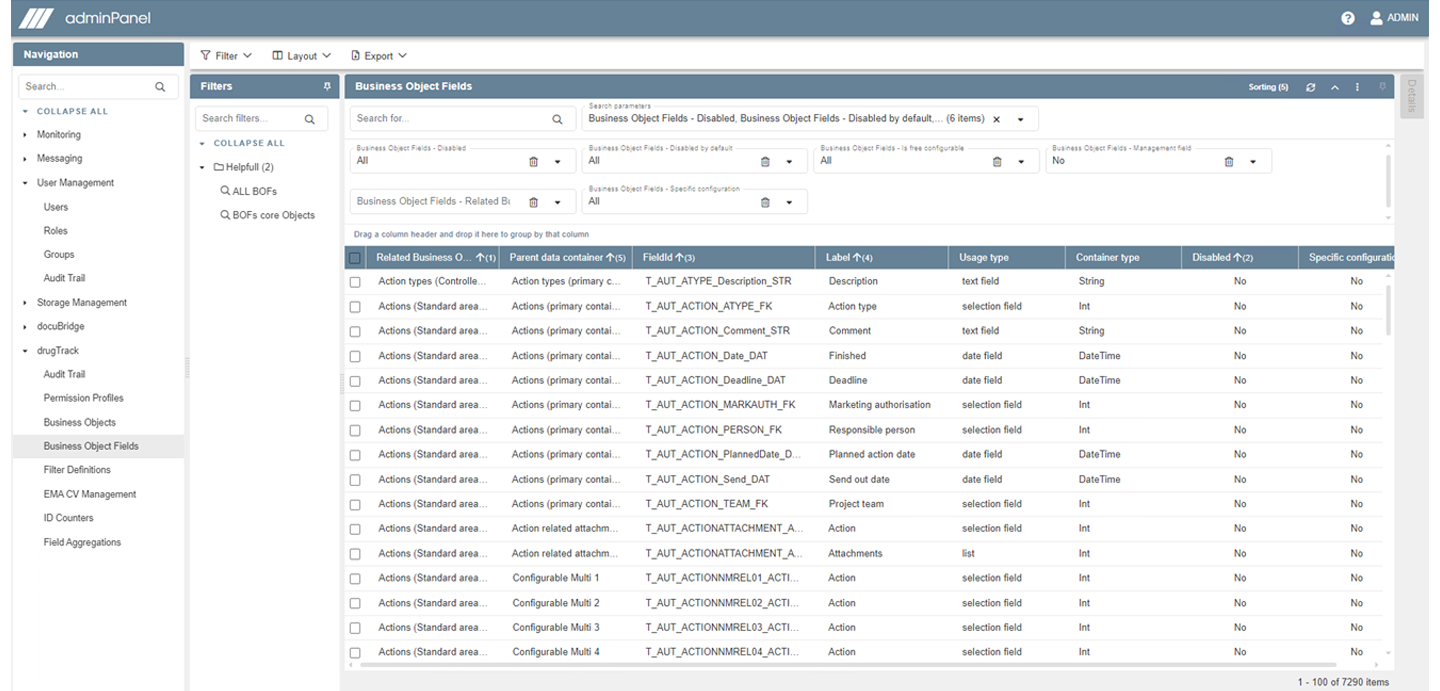
Prepare your data for the ISO IDMP standards: let drugTrack show you which fields are relevant for XEVMPD and which are relevant for SPOR/IDMP with colored tagging and popovers outlining the exact EMA requirements for these fields. Furthermore, you can filter by these tags as well as other fields such as ‘Mandatory’ or ‘Recommended’.
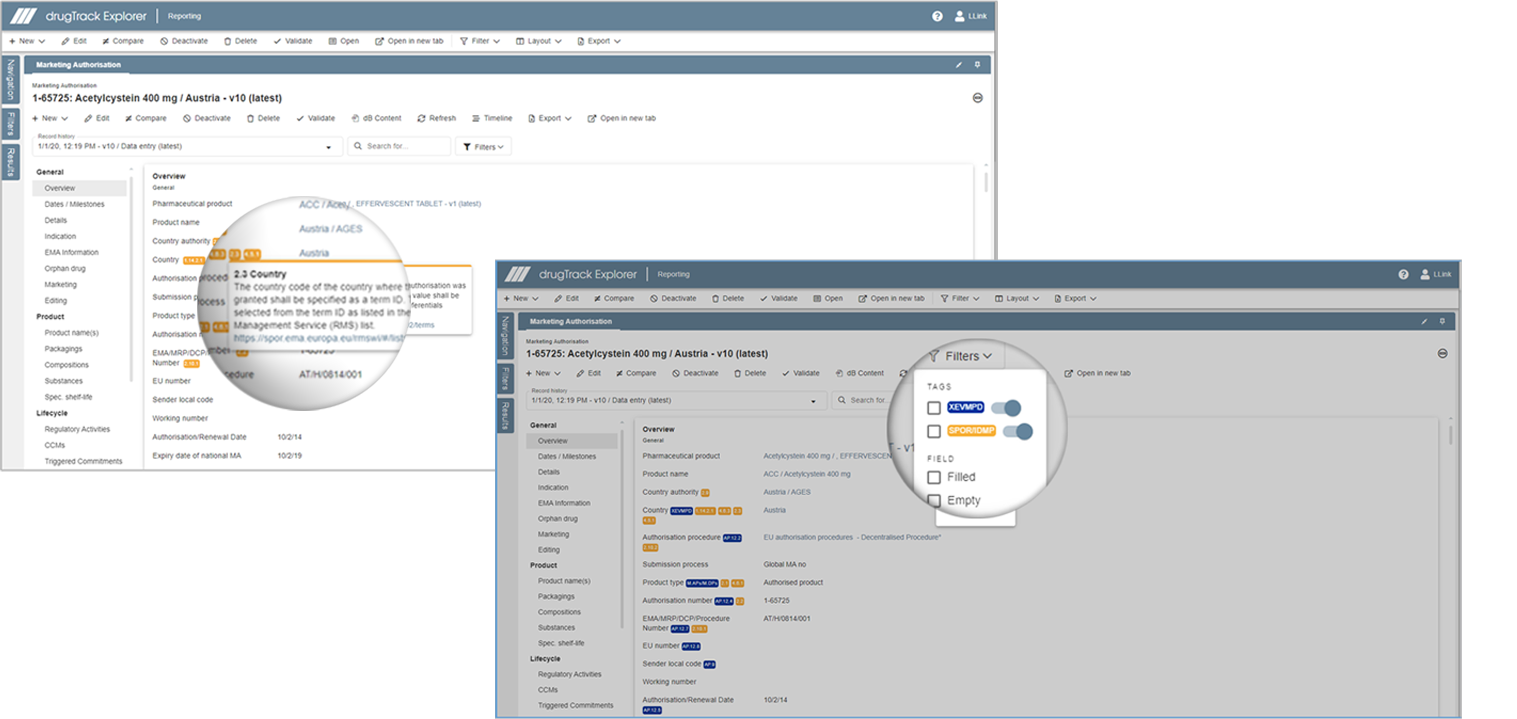
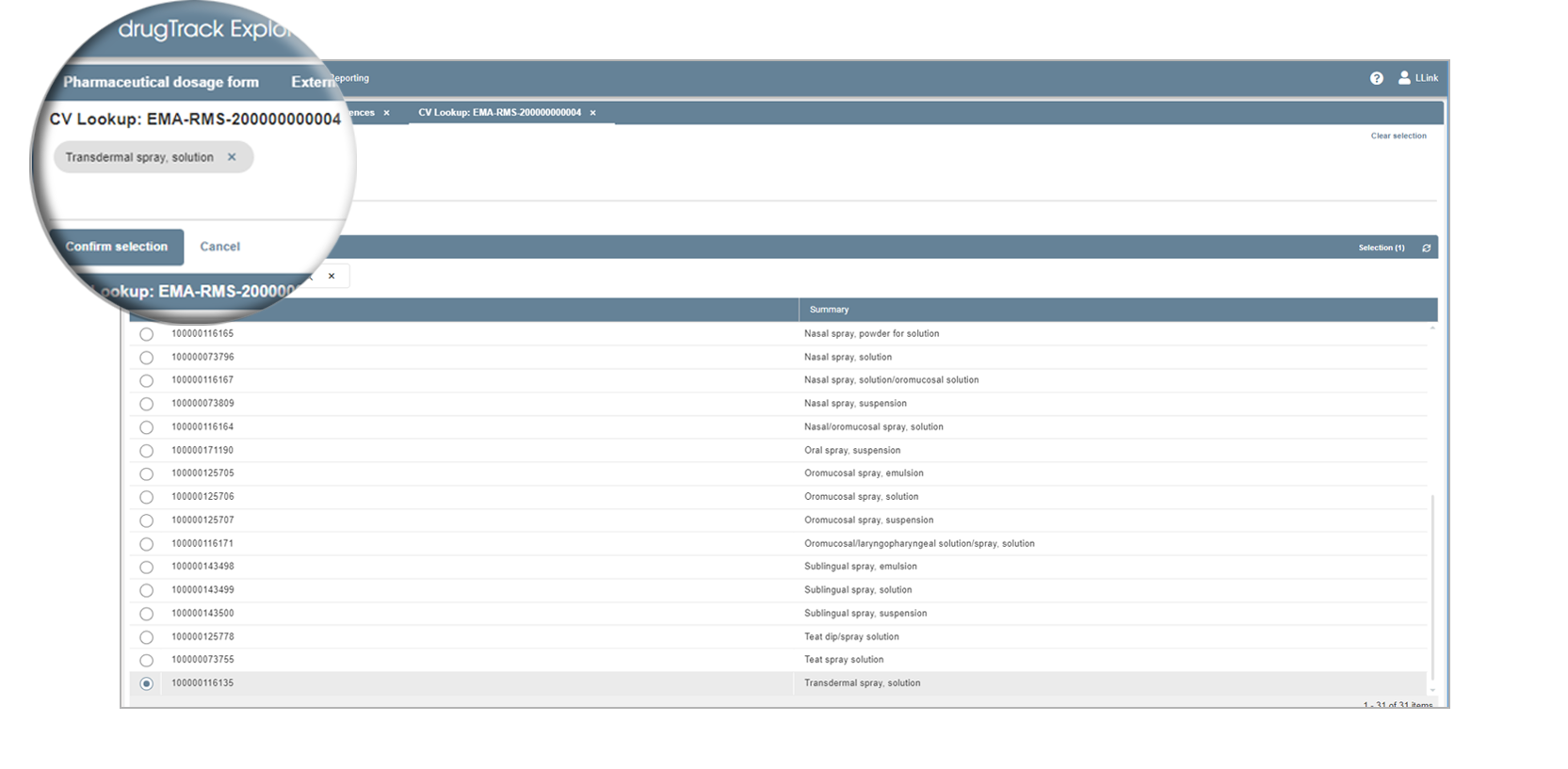
Update your controlled vocabulary
You can update your existing CV entries and link them to the new EMA RMS lists and OMS list. The SMS and PMS lists will be integrated as soon as the EMA publishes them.
Import EVWEB data
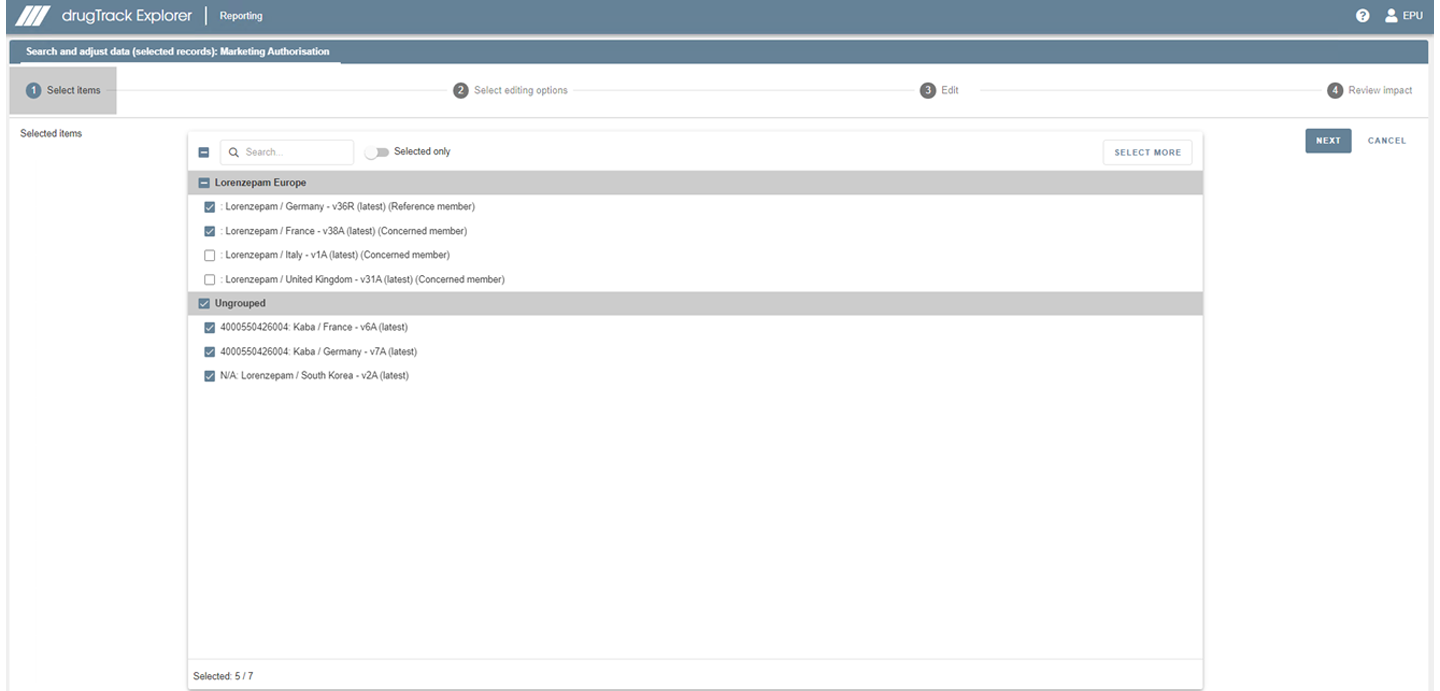
Our EVWEB tool allows you to easily import data which you have previously sent via EMA’s EVWEB. To become IDMP compliant you then only need to add the missing data.

IDMP Training
LORENZ offers IDMP Training to help you prepare for IDMP structured data submissions. We will provide you an overview of current implementation timelines, how EMA’s master data management SPOR plays a key role in the region’s pharmacovigilance, and regulatory processes and activities. We will help you understand the challenges of data quality and will conclude with the LORENZ framework and how to best help you (MAHs and Sponsors) during the transition period of the Article 57 databases (XEVMPD to PMS).