The new PMS enrichment feature enables the data enrichment process for the European Medicines Agency's Product Management System (PMS). drugTrack is among the first solutions to support this, allowing you to meet EMA's new PMS requirements directly within the system, without re-entering product information in the PMS UI.
How drugTrack can help you in submitting PMS enriched data to EMA
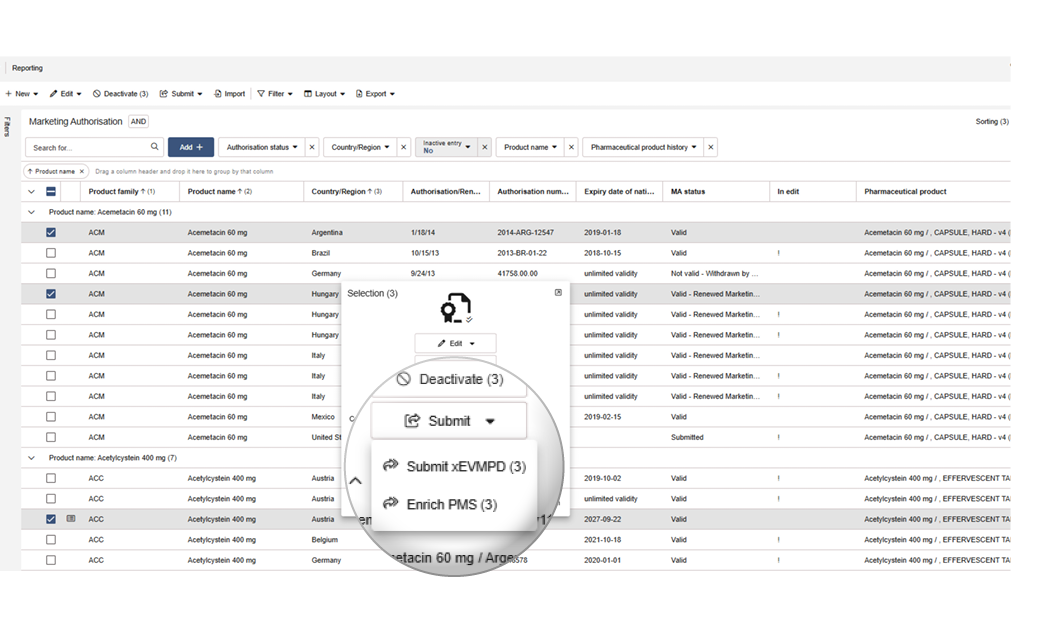
PMS enrichment directly in drugTrack
You can select one or multiple Marketing Authorisations to validate or enrich your PMS data, or perform both actions as needed. If a Marketing Authorisation is not yet approved or the user does not have the required permission, only the validation option will be available. This allows you to enrich PMS data efficiently and securely from within drugTrack.
Review and compare PMS data with ease
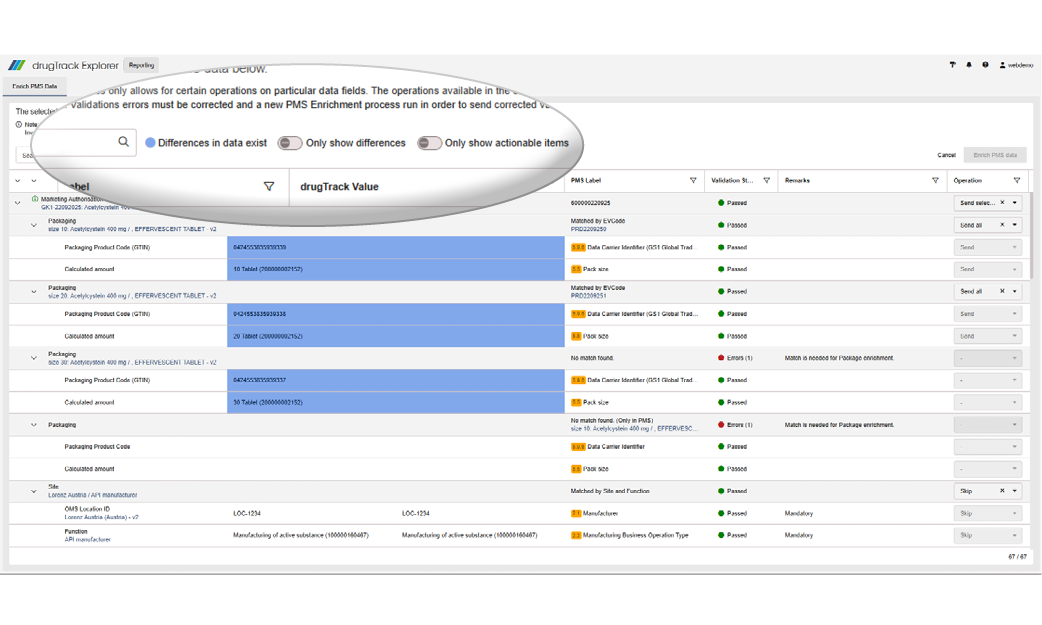
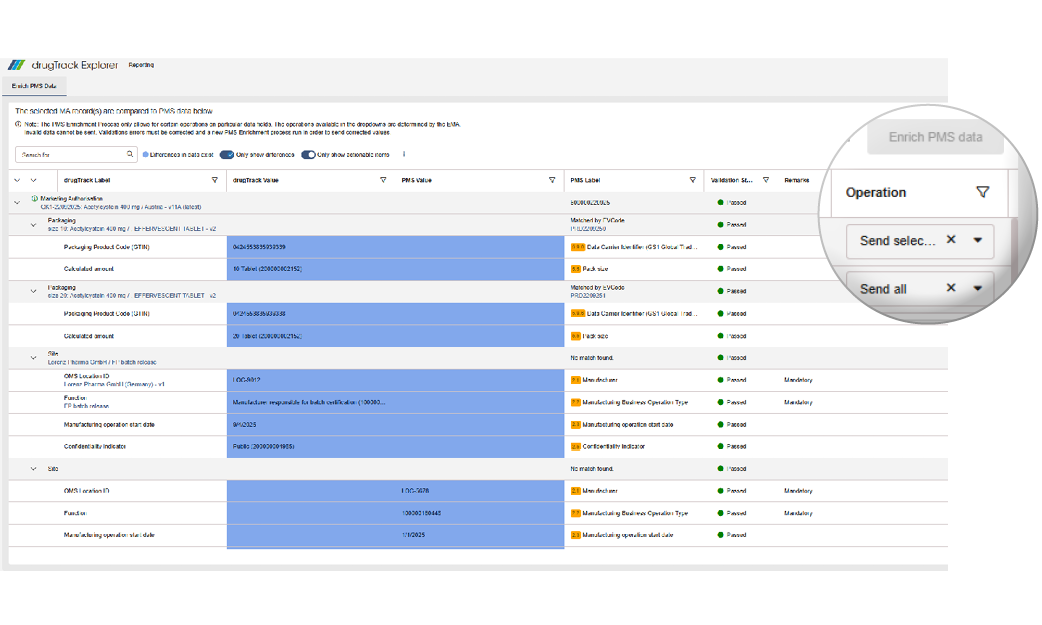
After selecting Enrich PMS, the new Enrich PMS Data screen displays all selected records in a clear table. Differences between your data and the PMS data are highlighted in blue for quick identification. You can filter, search, or export the table to focus on relevant information and keep your records consistent and up to date.
If activating the option to only show actionable items, the table only displays entries that can be sent or deleted. This compare view will also help you to verify the changes in PMS after your PMS enrichment, as the changes will be visible after a refresh of this page.
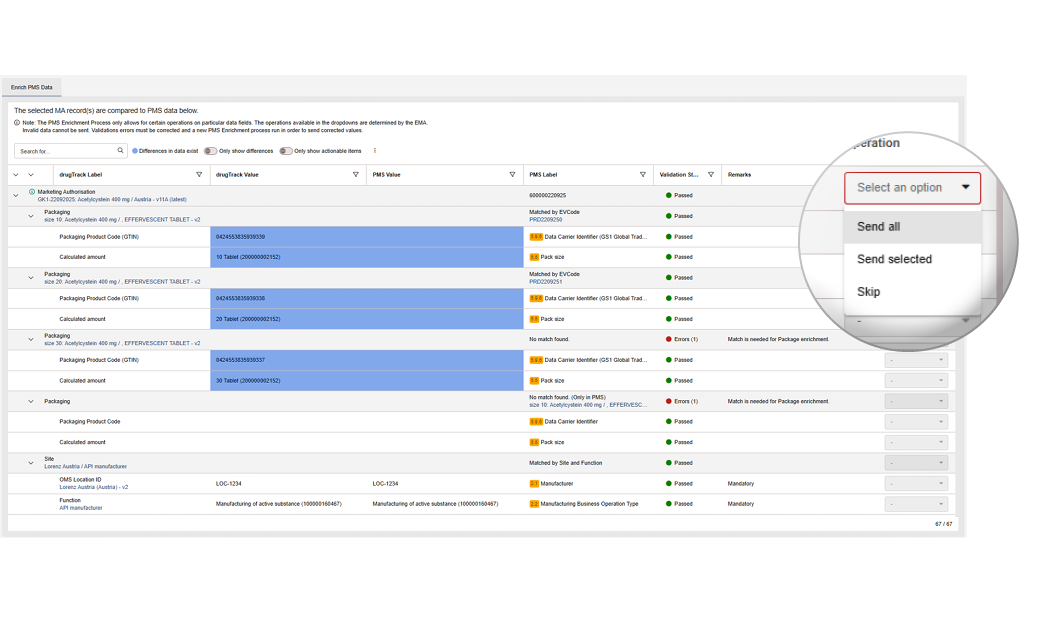
Full flexibility in how to handle each data entry
Choose how each information should be handled. You can decide which value is added, updated or deleted, giving you full control and ensuring accurate and compliant data.
Complete the enrichment process
After selecting how your data should be handled, start the enrichment process with a single click. drugTrack applies your chosen actions and updates the PMS data, keeping your product information complete and up to date.
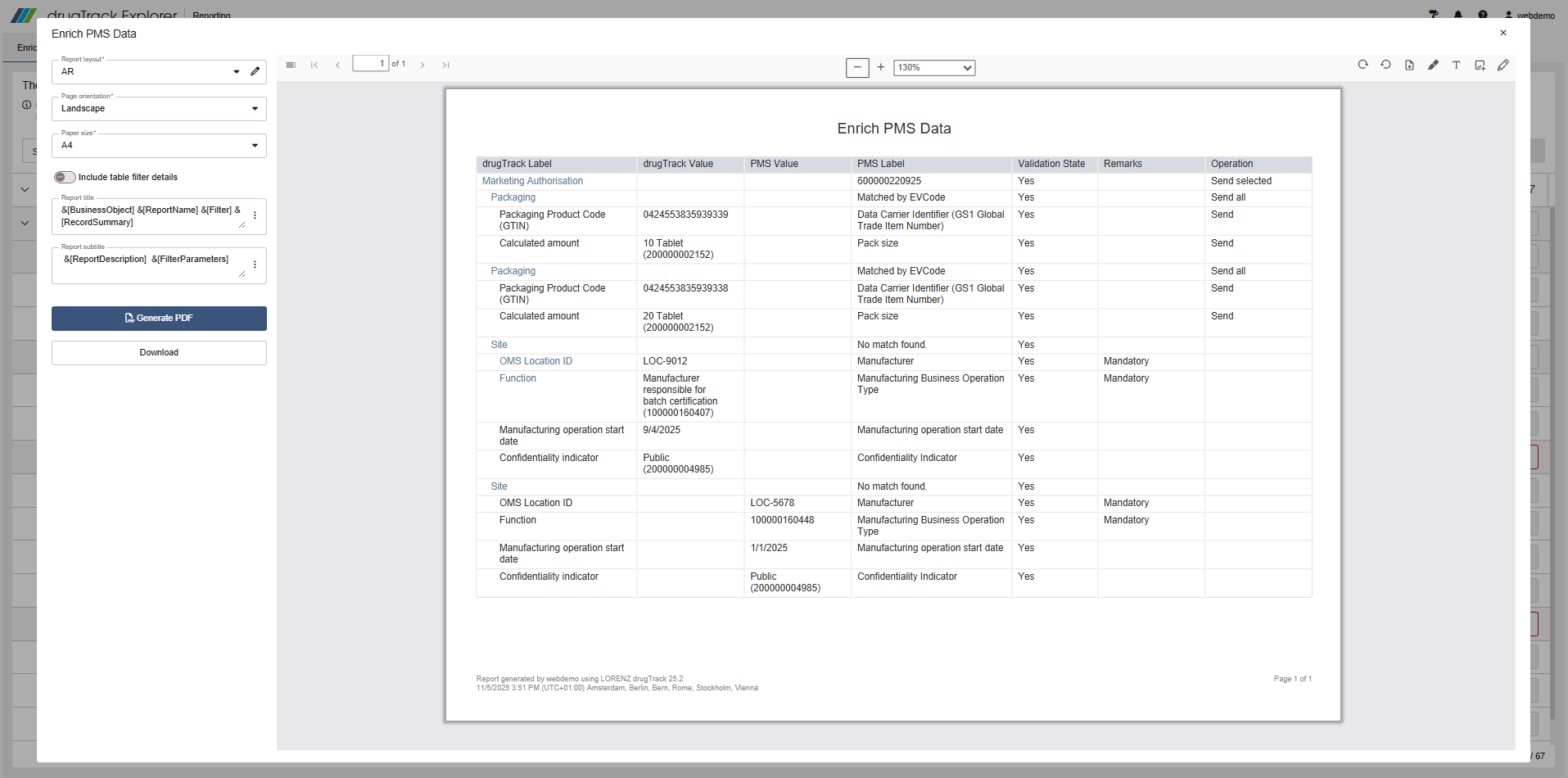
Create and share PMS enrichment reports
You can export an overview of the PMS Enrichment Compare screen to PDF or Excel to share with relevant stakeholders. The export follows the same familiar process used for other drugTrack reports, making it easy to document and communicate your PMS updates.
Optimizing Structured Data Submission for EU-IDMP: Package Level Submissions and PMS Enrichment activities for MAHs
EMA's implementation of IDMP in the EU: A Stepwise Approach
The European Medicines Agency (EMA) is adopting a phased implementation of the ISO IDMP standards in the EU with Product Management Service (PMS) Iteration 1. It focuses on a subset of authorised medicinal product data. This marks a crucial step toward harmonised, structured data use across regulatory procedures – supporting key initiatives like the European Shortages Monitoring Platform and integration of structured PMS data in the electronic Application Form (eAF).
We're proud to share that PMS API Write Access has been live since 25 September 2025 – and LORENZ was actively involved during the UAT phase, which successfully concluded at the end of August. In a significant milestone for the industry, LORENZ was one of the first to deliver PMS Enrichment via the API to EMA's PMS Production, marking real progress in the practical implementation of IDMP.
Watch this video to learn more about:
- The role of PMS in enabling data reuse across EU regulatory procedures
- Key deadlines and compliance requirements
- How to manage package-level submissions and enrichment activities efficiently
- Leveraging LORENZ's Gateway Submission module, which supports both the PMS Write API and XEVMPD for seamless, parallel submission processes
Stay informed. Stay compliant. Stay ahead – with LORENZ drugTrack.
About the speaker: Dr. Grace Ng-Krülle is a Senior Business Process Consultant and Lead RA Specialist. Since 2013 Grace helps customers navigate regulatory requirements by improving data quality and ensuring compliance. She is subject matter expert on electronic submissions of structured regulatory data for ISO-IDMP, specifically in the support of the European Network Portfolio.