Update on EU IDMP implementation
Posted on August 19, 2021
The year 2021 is a landmark one for IDMP implementation. After version 2.0 of the EU IDMP guide came out in February, version 2.1 of the implementation guide was released on 30 June. This now leaves only version 2.2 in the pipeline; currently anticipated in Q3 of 2021. After that, depending on the outcomes and learnings from step 1 of the TOM (Target Operating Model) implementation, version 3.0 of the EU IDMP guide will be published to support step 2.

Version 2.1 of the EU implementation guide now covers the following chapters and content:
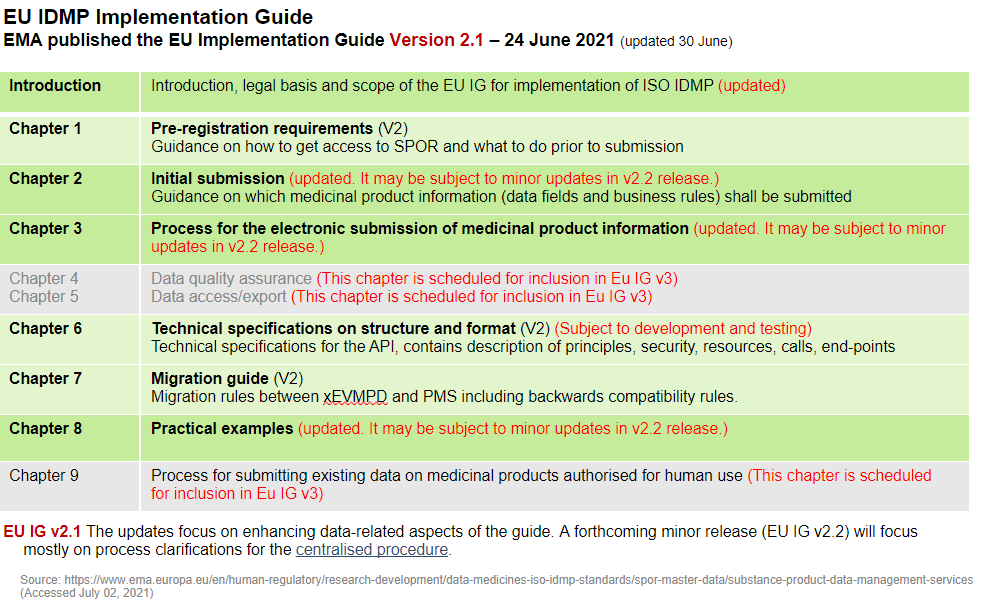
Recap of v2.0 of the EU IDMP implementation guide
The EU IDMP implementation guide v2.0 serves to support the European medicines regulatory network in preparing for step 1 of implementing the TOM. Building on a previous version, v2.0 provided:
- High-level principles of the target operation model for submitting and maintaining medicinal product data in the EU
- Further guidance on how to populate the PMS (Product Management Service) data elements
- The basis for medicinal product data exchange within the EU
During this period, the new FHIR format must comply with the Article 57 data submission requirements. FHIR or ‘Fast Healthcare Interoperability Resources’ is a draft standard from HL7 designed to allow the exchange of electronic health records. It will first become mandatory for CAPs and in the following steps for other procedures as well. In the future, FHIR could even possibly become mandatory at an international level. LORENZ is aware of this possibility and will be keeping a close eye on developments and will be making adjustments to our solutions as necessary.
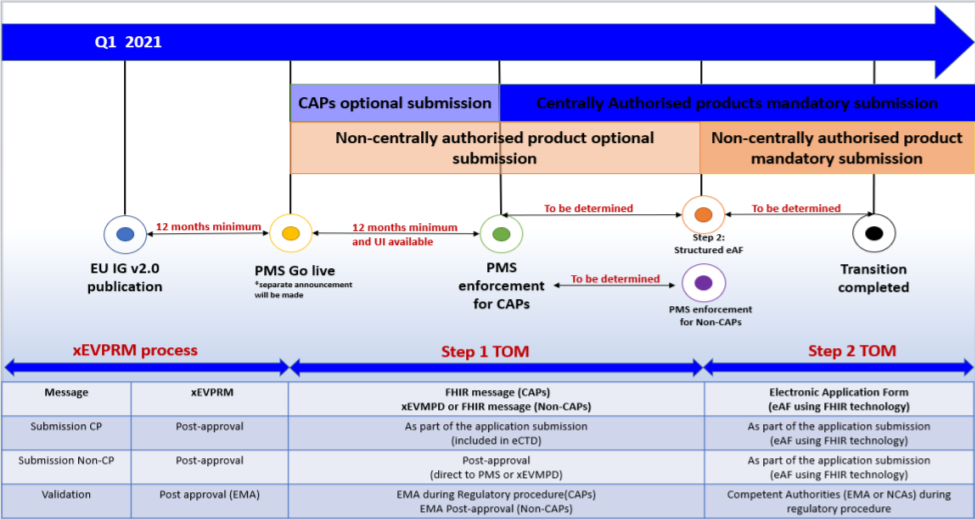
https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/product-management-services-pms-implementation-international-organization-standardization-iso_en.pdf (Retrieved 16 August 2021)
In step 1 of the TOM implementation which covers centralized procedures (CAPs), the FHIR format will be used within the actual procedure; i.e. the FHIR message will be part of the eCTD. The final FHIR message of the closing sequence is the one that will be uploaded to the PMS.
For non-centralized procedures (non-CAPs), the process is not yet fully defined. During this transition period, there will be a feedback loop between the PMS and XEVMPD (Extended EudraVigilance medic-inal product dictionary) database. During this time Marketing Authorisation Holders will have to submit their product information in FHIR format for CAPs in parallel to the XEVPRM format for non-CAPS.
After the transition period for the PMS iteration 1, submissions in IDMP format for CAPs will become mandatory. It was announced that there will be an official publication (EU IDMP implementation guide version 3.0) to cover the transition period between steps 1 and 2, which mark the start of the minimum 12-month countdown to mandatory CAP implementation.
New v2.1 of the EU IDMP implementation guide
The new update v2.1 brings:
- New data elements which will be required to support the PMS Target Operating Model
- Minor updates to the data elements that should be reported to PMS
- Details on the RMS lists
- Updated submission process and examples
As the EU IDMP implementation guide introduced new fields, it is now important for Marketing Authorisation Holders (MAHs) to analyze the impact of the new fields on their regulatory and organizational business processes, as well as their technological requirements. MAHs need to align the values from RMS with their internal data to assure they have the PMS data quality that is required when EMA migration from the XEVMP to PMS begins. With drugTrack, LORENZ aims to make the transition as simple as possible for you. Therefore, LORENZ is offering services to help you prepare for the upcoming IDMP (EU-SPOR) challenge.
In addition to these services, drugTrack itself comes with many helpful features to make the transition to IDMP easier. It lets you streamline existing data to make it compliant and improve the quality of the data. For example, if you have used ev-web before, you can easily import this data and then simply add the missing data to become IDMP compliant.
Once drugTrack is in place, you then have an efficient way to manage your data using functionalities such as grouping and volume editing. In addition, it offers you the possibility to add additional data with its configurable data model approach, enabling you to remain compliant with IDMP.
LORENZ drugTrack can also be part of your flexible RIM system by offering interoperability features with other LORENZ solutions as well as interfaces to third-party systems. With this you can choose exactly the capabilities you need.
This entry was posted in Regulatory Affairs News.
