Leveraging generative AI, LORENZ introduces further automation into the eCTD submission process. By combining technical compliance validation with AI-powered content checks, we ensure the technical accuracy of eCTD submissions while semi-automating the creation and consistency validation of document content. This integrated approach streamlines regulatory workflows, reduces errors, and enhances submission quality.
AI-Powered Content Validation
The Regulatory Content Validator verifAI revolutionizes submission document validation by leveraging generative artificial intelligence to create pre-defined content validation rules. Our automated rule generation, based on evolving regulatory guidelines, ensures documents meet accuracy, compliance, and completeness standards.
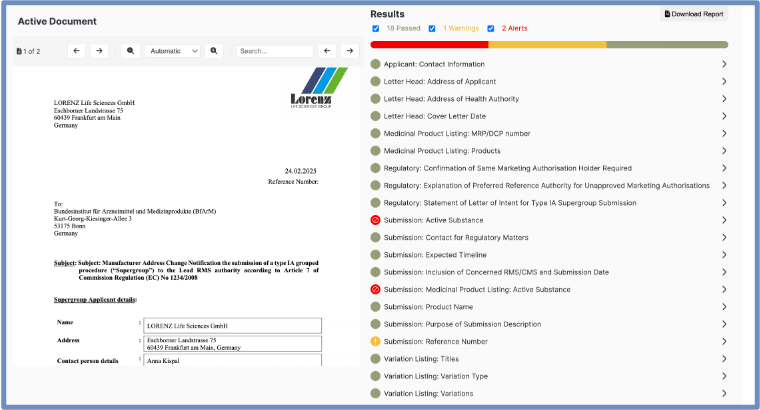
Users benefit from instant validation, whether authoring documents in Microsoft Word or uploading a complete collection of PDF files into the content validator. With a single click, verifAI generates a comprehensive validation report, identifying content inconsistencies, missing required information, and deviations from both historical submissions and industry regulations (e.g., FDA, EMA, ICH).
By automating content verification, verifAI significantly reduces review cycles, enhances document quality, and ensures every submission includes the necessary information saving regulatory teams valuable time and improving approval readiness.
LORENZ Regulatory Content Validator
Content quality
The Regulatory Content Validator ensures high-quality documents from the start by automating checks for completeness and structure. It guides authors during creation and flags missing or inconsistent sections – improving clarity, readability, and professional standards across all regulated content.
Content accuracy
By leveraging AI and historical document analysis, the Regulatory Content Validator identifies discrepancies and misalignments in text. It compares new content against past submissions, source data, and internal knowledge to ensure factual precision and eliminate errors before review.
Content compliance
The Regulatory Content Validator validates content against evolving regulatory, industry, and internal guidelines (e.g., FDA, EMA, ICH, ISO). With its automated rule generation engine, it adapts to specific requirements and instantly checks if all mandatory information is present – ensuring documents meet the highest compliance standards.
Take a closer look at verifAI
Benefits of verifAI - Regulatory Content Validator

Accelerated document authoring
Streamline the creation process to reduce the time spent on drafting and revising documents.
Shortened review cycles
Decrease the number of review iterations required to finalize documents, ensuring quicker approvals.

Reduced review time
Minimize the time needed for each review session by ensuring documents are submission-ready from the start.

Faster agency response submissions
Expedite the submission process, enabling faster responses to regulatory agencies.

Data consistency across submissions
Maintain consistency with historical submissions and ensure alignment with RIM data for seamless regulatory compliance.

First-time quality assurance
Enhance the readiness of submission documents, ensuring they meet quality standards right from the start.
Validation result of Content Validation