Lorenz Events
LORENZ Converge Conference & Optional Training Day
From: 11 Sep 2023 | To: 13 Sep 2023 | Location: United States
We're sorry, but LORENZ Converge 2023 is SOLD OUT.
We hope to see you at future LORENZ events!
If you still wish to participate, please contact us at marketing@lorenz.cc

Join us to hear from leading voices in the life sciences community, to exchange information, network, and share practical experiences with peers and colleagues. LORENZ Converge offers a variety of formats including plenary sessions, a range of Table Tutorials, and the opportunity to speak with our experts directly.

Learn about the newest and most loved features of docuBridge FIVE at our
training day.
Speakers


Digital Transformation – A Journey Towards a Data Driven Organization
Dorian Tisdale, Janssen Pharmaceutical Companies of
Johnson & Johnson

Enhancing Local Affiliate Engagement for Efficient Global RIM Programs
Steve Gens, Gens & Associates

Regulatory and Scientific Collaboration via Cloud Platforms
Vada A. Perkins, Bayer Pharmaceuticals

docuBridge & Veeva: From a Company's Regulatory Need for Global Products to Health Authority Submissions
Dr. Max Nattermann, Bayer AG

Stop Emailing Me, Annotate Me Instead…Using Annotation Feature for Submission Review
Rachelle Butt, United Therapeutics Corporation







Simplifying the Publishing and Maintenance of Investigational Medicinal Product Dossiers (IMPDs)
Swathi Pandhiti, MMS Holdings Inc.


Stop Emailing Me, Annotate Me Instead…Using Annotation Feature for Submission Review
Ginny Smith, United Therapeutics Corporation

Business Benefits, Approaches and Insights Gained from Regulatory Intelligent Automation Implementations
Cary Smithson, PharmaLex

Building Grouped Submissions: Tips for Success
Allison Steffen, WAYS Pharmaceutical Services

Reusing EU Marketing Authorisation Application in the US and Vice Versa
Claudia Bernal-Vallejo, LORENZ Life Sciences Group

Functions & Permissions: Differences Between Groups & Roles
Sebastian Hagedorn, LORENZ Life Sciences Group

Simplifying Submission Handling and Sending: Introducing the Submission Repository
Anna Kispál, LORENZ Life Sciences Group

Keynote
Raoul-A. Lorenz, LORENZ Life Sciences Group

Practical Experiences from US FDA eCTD 4.0 Pilot
Jessica Lemley, LORENZ Life Sciences Group




LORENZ MetaSync – Flexible Metadata Synchronization
Dr. Katharina Schmitz, LORENZ Life Sciences Group

How LORENZ RIM Supports Registration Tracking & Reporting
Matthias Spiller, LORENZ Life Sciences Group

Conference Schedule
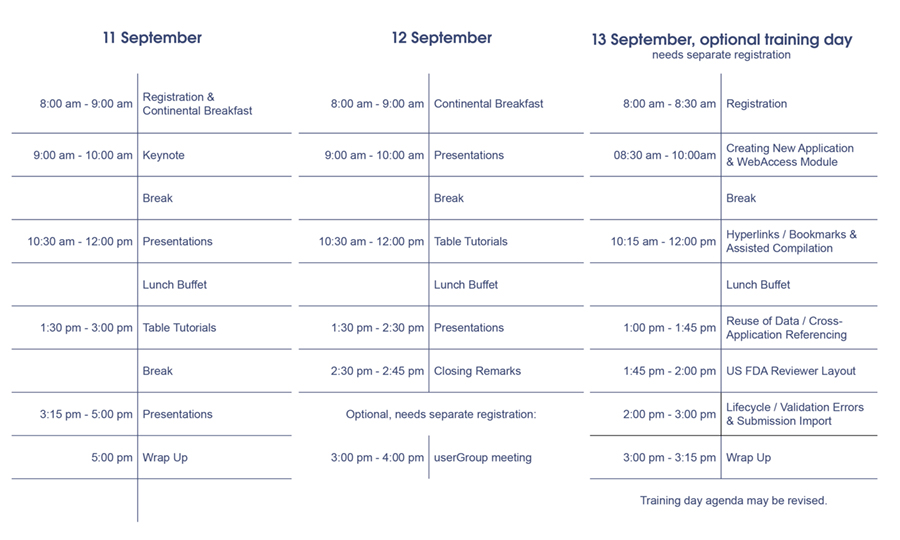
* Following the official portion of the conference, you also have the opportunity to participate in a meeting of the docuBridge userGroup. This group was founded by two LORENZ customers with the aim of gathering best practices for efficient regulatory publishing in docuBridge. At the same time, it provides a forum for the discussion of current regulatory guidelines. For more information on the userGroup please click here.
We are looking forward to seeing both familiar and new faces!

Conference location
The Otis Hotel Austin
1901 San Antonio St,
Austin, TX 78705, USA.
To book your room at The Otis Hotel, please click here.
Please note that the number of rooms is limited.
Conference fees (US$):
- Industry: $1,200 (no credit card payments)
- Regulatory Authorities: Free
- Speaker & Table Tutorial Leader: Free
- Optional Training Day for docuBridge FIVE: $ 700 (no credit card payments)
Cancellations
For cancellations received on or before August 11, 2023, you will receive a 50% refund.
For cancellations received after August 11, 2023, you will be charged the full amount.
Please stay tuned here for more detailed information on the agenda, speakers and session times at LORENZ Converge.
Email contact: www.lorenz.cc/email



